6. Therapeutic Vaccines
For many years, vaccines have been used to successfully prevent devastating infectious diseases such as smallpox, measles and polio and more recently human papillomavirus (HPV) and pneumococcal infections. These public health triumphs illustrate the major contributions that vaccines have made in saving countless lives around the world. Nevertheless vaccines are not only for preventing infectious diseases, some help the body fight a range of illnesses by activating the immune system to recognise and attack disease. Therapeutic vaccines have been the subject of massive R&D efforts, both from academics and industry, most notably biotech companies. Since 2010, more than 800 publications have addressed the topic21. In 2013, Science Magazine designated cancer immunotherapy as “Breakthrough of the Year”22.
In 2010, a new cancer vaccine, Provenge (Dendreon), for the treatment of prostate cancer was approved in the United States, and many more immunotherapeutic vaccines are in development. In 2014, the EMA accepted Dasiprotimut-T BiovaxID marketing authorisation application for the treatment of non-Hodgkin’s follicular lymphoma in patients who have achieved a first complete remission.
Today, the pipeline for therapeutic vaccines has grown to an estimated 470 products. The vaccines in development cover more than 70 different conditions. There is a strong focus on areas of high unmet need, such as vaccines against cancer and infectious diseases, accounting for 55% and 24% of the pipelines respectively. Products in late stage development include potential treatments for multiple cancers, infectious diseases (HIV, Hepatitis…), allergies, diabetes, and addictions. Therapeutic vaccine companies must choose among a variety of delivery systems, immunopotentiators/adjuvants, product technologies, and production platforms — with many of these factors being unique to therapeutic vaccines. Companies are also developing new immunological assays for the identification and validation of novel vaccine candidates (MedTrack, 2015). Today there is no clear single definition of a therapeutic vaccine, however in general today’s therapeutic vaccines mediate their effect through in vivo induction or amplification of the antigen-specific host immune response. Mmunotherapeutic products thus comprise a broad range of approaches: antibodies, peptides, proteins, nucleic acids, immune cells (i.e. dendritic cells, Tcells…) or stem cells, tumour antigen specific proteins and gene therapy products. Often, the therapeutic regime includes different combinations of these tools (e.g. antivirals to reduce viral loads, followed by therapeutic antibodies to maximise immune responses and minimise immune exhaustion, and a prime boost vaccine for chronic infection).
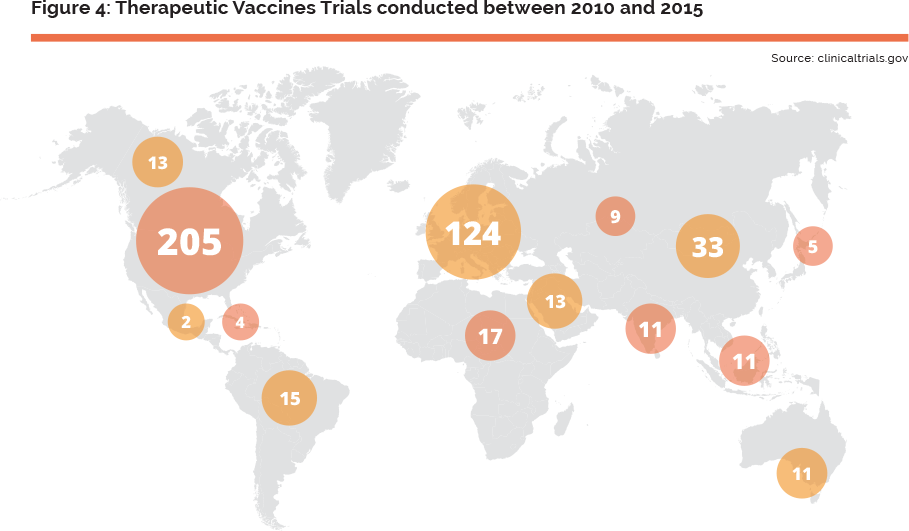
Therapeutic vaccines are intensely explored in the area of oncology (54% of therapeutic vaccines clinical developments in 2012), as can be seen in the recent major deals between BMS and Bavarian Nordic23 and Roche and Immatics biotechnologies (November 2013), and Boeringher Ingelheim and CureVac (2014), for such innovations. This perspective calls for the need for a European agenda on this type of vaccines’ R&D. T-cell–based of therapeutic cancer vaccines, combined to standard care therapies and/or immune checkpoint blockades, are considered to be the most relevant therapy to tackle cancers. Such combinations are overcoming both efficacy issues by targeting specific cancer biomarkers and safety issues by limiting toxicity induced by standard of care therapies and immune- related adverse events induced by immune checkpoint blockades. Therapeutic vaccines are also being studied in a number of therapeutic areas outside of oncology. Research spans from Parkinson’s and Alzheimer’s disease to multiple sclerosis, rheumatoid arthritis, diabetes and Crohn’s and celiac disease. Many of these activities are rather driven by market size or business potential than scientific advancements, e.g. developing a therapeutic vaccine against coeliac diseases is preferred to an orphan disease such as myasthenia gravis, since the former is more attractive to venture capitalists than the latter. Considering the progressive ageing of the European population and increasing burden of chronic diseases, the application of therapeutic vaccines to these diseases could clearly be an area of great potential in support of future health policies of the European Union. Despite these promising approaches, many hurdles still exist, and if the number of clinical trials is used as the metric, Europe is lagging behind the US, (see Figure 4 on the next page).
As highlighted by the IPROVE consulting panel, on top of high occurrence of undesired outcomes or unsatisfying efficacy, the transition of clinical trials in therapeutic vaccines to the next stage or to submission is challenging in respect to time, for the following reasons:
- Organisational capacity and capability for development: therapeutic vaccines research is often conducted in
a university spin-off or is dependent on one inventor, either of which has limited organisational capabilities. - Financing issues: acquiring funding in the early
Stages and retain sustained financial support over the development phases is a particular hurdle. Proof-of- concept takes at least 5-8 years. Furthermore, efficacy studies in therapeutic vaccines take a long time, e.g. it takes 7-8 years to demonstrate the efficacy of a cancer vaccine to reduce cancer recurrence. - Re-orientation of programmes: founders and board members of companies investing in the field might have different ideas on the direction of further development; consolidating a real strategy can take years.
As a result, an R&D agenda is needed for the R&I of therapeutic vaccines. From a purely R&D perspective, therapeutic vaccines share most of the challenges and gaps along the value chain that occur in prophylactic vaccines, described in the roadmap. However, on top of these, the workshop dedicated to therapeutic vaccines highlighted three key additional types of challenges specific to therapeutic vaccines that are discussed separately in the sections below.
GAPS & CHALLENGES
A first gap is the lack of a therapeutic vaccines network at european level.
During the consultation, the lack of a therapeutic vaccines network has been stressed as a critical gap that would need to be addressed in order to drive innovation in this field. Currently, over 70% of clinical therapeutic vaccine candidates are being developed by biotech or small to mid-size pharma companies, which often lack the broad capabilities and long-term expertise in both technology and therapeutic areas to fully drive the development. Overcoming this national fragmentation is essential to providing therapeutic vaccines with the necessary market perspective needed to develop products for global medical needs. A lot of research is conducted on adjuvants or delivery mechanisms but the community is often not aware of the ongoing research of all the various groups. There is a tradition of collaborating projects in prophylactic vaccines; e.g. ADITEC24 where adjuvants from different companies are shared and a head to head comparison of different antigens with the same adjuvants is performed, or TBVAC202025 where candidate vaccines are compared head-to-head before going into further clinical development. Similar partnerships and large projects in the field of therapeutic vaccines are also needed.
The second main challenge appears to be that the applicable regulatory framework to the development of therapeutic vaccines is considered to be unclear in europe today.
Currently, at EU level, the Regulation (EC) No 1394/2007 of the European Parliament and of the Council on advanced therapy medicinal products and amending Directive 2001/83/ EC and Regulation (EC) No 726/2004 regulates the area of therapeutic vaccines and is split into gene therapy, somatic cell therapy and tissue-engineered products. The Guideline on the evaluation of anti-cancer medicinal products in
man provides some guidance on therapeutic vaccines for oncology, but guidance is missing for other indications. While the European Medicines Agency considered that it would be difficult and not warranted to have a regulatory guideline that could cover all possible clinical developments for therapeutic vaccines, some of the stakeholders consulted suggested to work on a better legal definition of “therapeutic vaccines” around one comprehensive concept to simplify the regulatory environment for the field. The US’ Clinical Considerations for Therapeutic Cancer Vaccines’, (October 2012) is taken as an example of good practice specifically in the field of cancer vaccines. Stakeholders consider that having clarity on the regulatory side would allow competing on the same basis and in a levelled regulatory environment across regions. The EMA recommended interacting early on with regulators on the basis of specific plans, when starting vaccine development in new areas, especially since some consultations are free for certain stakeholder categories.
Finally, there is a gap in creating a reliable funding environment appropriate for companies developing therapeutic vaccines.
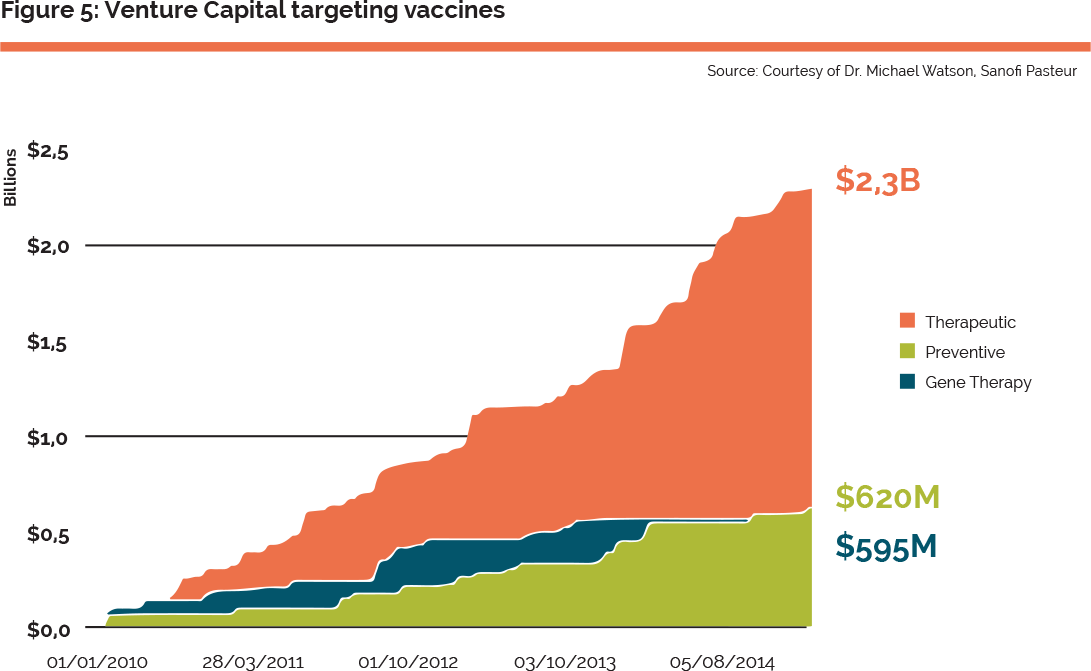
This is a long, complex and expensive process. It often starts with the translation of fundamental research to biotechnology start-up companies, often funded by venture capital. There is some indication that the interest of venture capitalists is increasingly in therapeutic rather than prophylactic vaccines. The EC’s Horizon 2020 programme, Juncker’s investment plan and the programmes of the European Investment Bank – in particular the “Infectious Disease Finance Facility”26 – are all expected to contribute positively to the situation in Europe. However, the consensus from the workshop participants was that Europe lags behind the USA in its ability to structure and fund biotechs and university spin-offs in the area of oncology. For example 70% of all VC investments are led by US funds and there is no EU equivalent of the US’s National Cancer Institute (NCI), which plays the role of a catalyst for therapeutic cancer vaccines. As for prophylactic vaccines, there was a consensus that, beyond funding considerations, Europe could benefit from a more connected ecosystem for therapeutic vaccines. Examples in the US include networks of academics, innovative SMEs and VCs often geographically co-located. In Europe networks are only starting to emerge in a more or less formalised way in certain countries or regions (e.g. in Belgium and Netherlands). In this regard a transversal project could help to increase the momentum of this market, stimulating cross-fertilisation across the major European players in this area.
RECOMMENDATIONS FOR EU LEVEL ACTION
In the end, the consultation resulted in four main recommendations:
Firstly, the workshop concluded that the field of therapeutic vaccines as a whole should be moved up within research programming and funding priorities. The consultation also concluded not to prioritise within the field (rare diseases vs. severe, more frequent diseases, as well as private vs. public prioritisation).
In the short term, a task force should be set up involving all stakeholders active in the field of therapeutic vaccines at eu level, in order to develop a collaborative network.
Such a collaborative network would benefit advances in therapeutic vaccines in 4 ways:
- Exchange findings and potentially bundle efforts, exchange best practice, successful and unsuccessful approaches, and sharing know-how and technology
- Make it easier for big pharmaceutical companies to identify interesting new research developments and allow them to support initiatives financially or capacity-wise
- Help with the understanding of underlying disease mechanisms and the interaction of disease-causing agents with the human immune system
- Design and perform multi-centre clinical studies
This in turn is likely to lead to shorter developmenttimes and lower attrition of viable projects.
Thirdly, the eC could facilitate close collaboration between therapeutic vaccines developers and regulatory agencies in order to address the regulatory challenges.
- In the short term, EC could support the organisation of regular workshops between therapeutic vaccine developers and regulatory agencies, such as the Committee for Advanced Therapies’ workshop on gene-therapy or the Paul-Ehrlich Institute’s workshop on viral vectors, in order to promote the exchange of information and discussion with the therapeutic vaccine community.
- In the longer run, such discussions could aim at clarifying the regulatory environment. Such evolution would require regulators to assess, where relevant, the feasibility of developing EU wide guidance for the development of therapeutic vaccines; for instance this could be in the area of cancer therapeutic vaccines akin to the US FDA model, in view of fostering an equally competitive environment from a regulatory perspective for new thriving research.
In the end, the consultation resulted in four main recommendations:
Although funding programs exist at EU level and the efforts to facilitate SMEs’ inclusion have been acknowledged, four levers have been identified to create a supportive financial environment:
1. Bridging the gap between research and market: it is key to concentrate EC policies for promoting availability of grants and risk capital on innovative researchers mainly for early stages (pre-clinical), but also during development stages.
2. Creating Efficient Financial Markets: Taking the different financial markets of EU Member States into consideration, governments need to assure that their financial markets operate efficiently so that most deserving firms would have access to financing and successful ones would adequately be rewarded.
3. Government policies to Improve Equity Financing: Access to funding via similar or new vehicles has to be improved in Europe. The Juncker Investment Plan & the European Investment Bank should play a pivotal role in implementing an innovative financing strategy.
4. Lower risk perception: Funding opportunities could be increased by lowering the risk perception of investors. A big incentive for investors would be a possibility to have an agreement with payers.
| Therapeutic Vaccines |
| GAPS & CHALLENGES | Recommendations | |
| Organisational challenges: no therapeutic vaccines’ network at eu level | Provide greater visibility and investment in the field of therapeutic vaccines as a whole within EU research programming.
|
|
| Regulatory challenges: eu regulatory framework is unclear | Foster early dialogue with regulatory bodies
|
|
| Financial challenges: gap in creating a reliable funding environment appropriate for companies developing therapeutic vaccines |
|
22 Cancer immunotherapy, (Dec. 2013), Science (vol.342)
23 Exclusive Agreement with Bavarian Nordic for PROSTVAC
24 http://www.aditecproject.eu
25 http://www.tbvi.eu/projects/tbvac2020.html